is the food code fda a administrative law
Implementation of the FDA Food Code also supports many of the food safety objectives of Healthy People 2020 the comprehensive. Senator from New York.
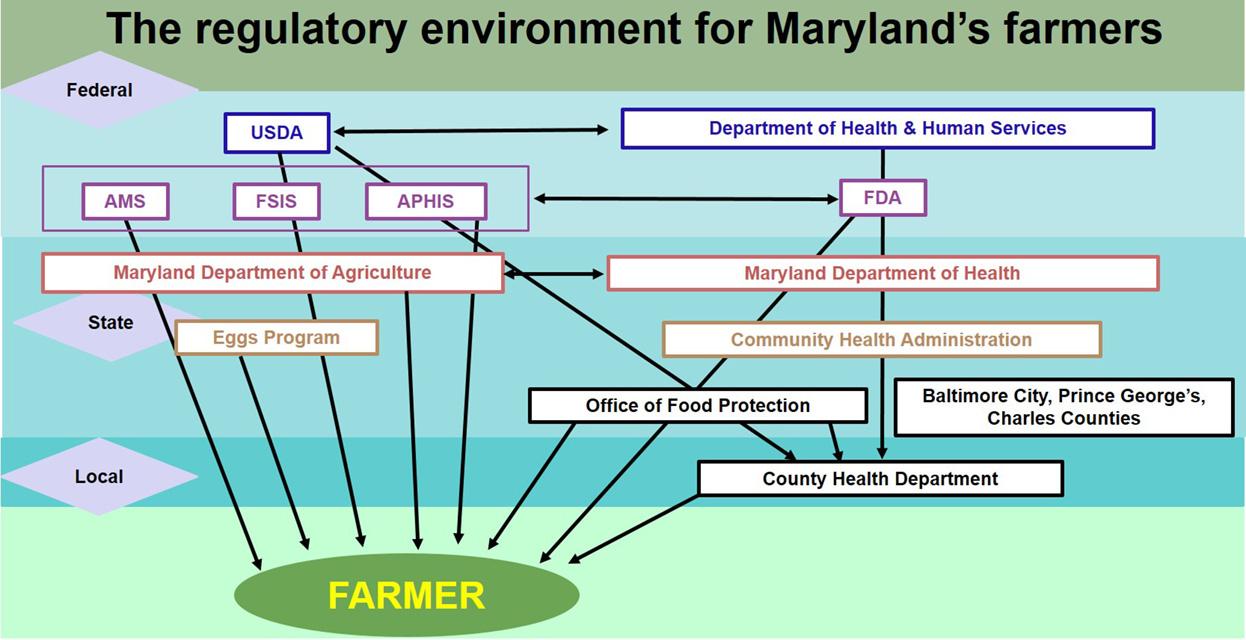
Agencies And Jurisdictions University Of Maryland Extension
A goal of the Food and Drug Administrations FDA Retail Food Safety Initiative is to encourage widespread uniform and complete adoption of the FDA Food Code Adoption of the Food Code.

. Title 21 of the CFR is reserved for rules of the Food and. The United States Federal Food Drug and Cosmetic Act abbreviated as FFDCA FDCA or FDC is a set of laws passed by Congress in 1938 giving authority to the US. The 2017 Food Code is the most recent full edition published by FDA.
The FDA Food Code is currently updated every four years with the last full update in 2013. That are shared by both the Food Establishment and Food Processing Plant operations eg refrigeration units dressing room and toilet facilities food equipment water and waste systems pest control might be subject to similar regulatory requirements. For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR.
Summary of Changes in the Food Code 2017. The FDA Food Code is not federal law. The FDA is tasked by law to prescribe standards guidelines and regulations with respect to information advertisements.
The law applied safety and effectiveness safeguards to new devices. Food and Drug Administration FDA publishes the Food Code. The law grants the FDA a number of new powers including mandatory recall authority which the agency has sought for many years.
Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the retail and food service segment. Supplement to the Food Code 2017 PDF. The Food Code is guidance representing FDAs current thinking and is a model on safeguarding public health and ensuring food is unadulterated and honestly presented when offered to the consumer.
Subsequently question is when was the FDA Food Code last updated. Today the FDA regulates 1 trillion worth of products a year. Copeland a three-term US.
1055 Separation of functions. Provided that every food package bears a code or mark to identify the processing plant. A The food additive is the refined hydrocolloid.
Subsequently question is who writes the FDA Food Code. Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound technical and legal basis for regulating the retail and food service segment of the industry restaurants and grocery stores and institutions such as nursing homes. The Federal Food Drug and Cosmetic Act FDC Act is a federal law enacted by Congress.
Mandate of the Food and Drug Administration. Food and Drug Administration FDA publishes the Food Code a model that assists food control jurisdictions at all levels of government by providing them with a scientifically sound. 500KB The Food Code is a model for safeguarding public health and ensuring food is.
The FDA Food Code is not federal law. The Philippines Food and Drug Administration FDA issued Administrative Order No. Subpart G - Gums Chewing Gum Bases and Related Substances.
Food and Drug Administration FDA to oversee the safety of food drugs medical devices and cosmeticsA principal author of this law was Royal S. If for example the FDA passes new rules that affect your business you. The FDA Food Code is based on recommendations from the Conference for Food Protection.
For the most up-to-date version of CFR Title 21 go to the Electronic Code of Federal Regulations eCFR. It is up the agencies that have responsibility for food safety to either adopt or adapt the FDA code to their own jurisdiction. An administrative law attorney specializes in administrative procedures at the federal andor state level.
PART 172 -- FOOD ADDITIVES PERMITTED FOR DIRECT ADDITION TO FOOD FOR HUMAN CONSUMPTION. It is the FDAs best advice for ways to ensure that food at retail and in foodservice is safe properly protected and presented. The laws administered by the Commissioner or the laws administered by the Food and Drug Administration means all the laws that the Commissioner is authorized to administer.
Some examples of administrative agencies include the Food and Drug Administration FDA the Internal Revenue Service IRS and the Federal Trade Commission FTC. The information on this page is current as of Jan 06 2022. The FDA Food Code is not federal law.
The food additive carrageenan may be safely used in food in accordance with the following prescribed conditions. F The term presiding officer means an administrative law judge qualified and appointed as provided in the Administrative Procedure Act 5 USC. CFR - Code of Federal Regulations Title 21.
A This section applies to any matter subject by statute to an opportunity. Admiralty and Maritime Law. 2014-0030 or otherwise known as the Revised Rules and Regulations.
Regulatory hearing before the Food and Drug Administration means a hearing conducted under part 16. Secretary means the Secretary of Health and Human Services. This database includes a codification of the general and permanent rules published in the Federal Register by the Executive departments and agencies of the Federal Government.
It supports the Food Safety Working Group created by the Obama administration to modernize statutes that require effective sanitation and preventive controls in food establishments. 3720 or otherwise known as the Food Drug and Cosmetic Act is the law creating the Food and Drug Administration FDA which is under the Office of the Secretary of the Department of Health. The FDA Food Code is not federal law.
It and other federal laws establish the legal framework within which FDA operates. It is the FDAs best advice for ways to ensure that food at retail and in foodservice is safe properly protected and presented. The Food Safety Modernization Act FSMA was signed into law by President Barack Obama on January 4 2011.
The FSMA has given the Food and Drug Administration FDA new authority to regulate the way foods are grown harvested and processed. It represents FDAs best advice for a uniform system of provisions that address the safety and protection of food offered at retail and in food service. G The term proceeding means all actions involving a hearing commencing with the publication by the Administrator of the notice of proposed rulemaking or the issuance of an order to show cause.
The Food Code is intended to apply to food establishments. Food Code 2017 PDF. Local Government Election Law and Administrative Law.
The FDA Food Code supports many key national food safety efforts.

Food Ingredient Packaging Terms Fda

Code Of Federal Regulations Title 21 Food And Drugs Parts 800 1299 Pdf Centerwatch

Researching Administrative Law Ppt Video Online Download

The Fda Food Safety Modernization Act P L 111 353 Everycrsreport Com

Introduction To Administrative Law Organization And Control Of

Researching Administrative Law Ppt Video Online Download

Enforcement Of The Food Drug And Cosmetic Act Select Legal Issues Everycrsreport Com

Food Safety First Principles For Food Handlers English Non Ansi Nrfsp

Regulations Administrative Materials Food Drug And Cosmetic Law Research Guide Guides At Georgetown Law Library

What Is Law Coercive Nature Of Law I E Not Voluntary Rules Of The Sovereign Legitimate Authority Backed By Force Problem Who Is The Ppt Download
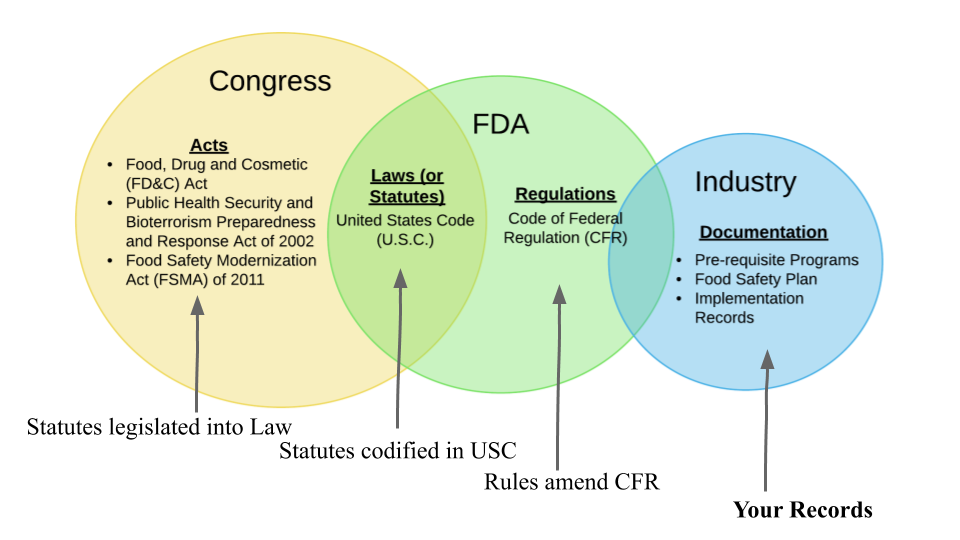
Introduction To Food Safety Compliance Terms Food Safety Guides

Food Product Labeling Requirements Of The Fda Law Firm In Metro Manila Philippines Corporate Family Ip Law And Litigation Lawyers
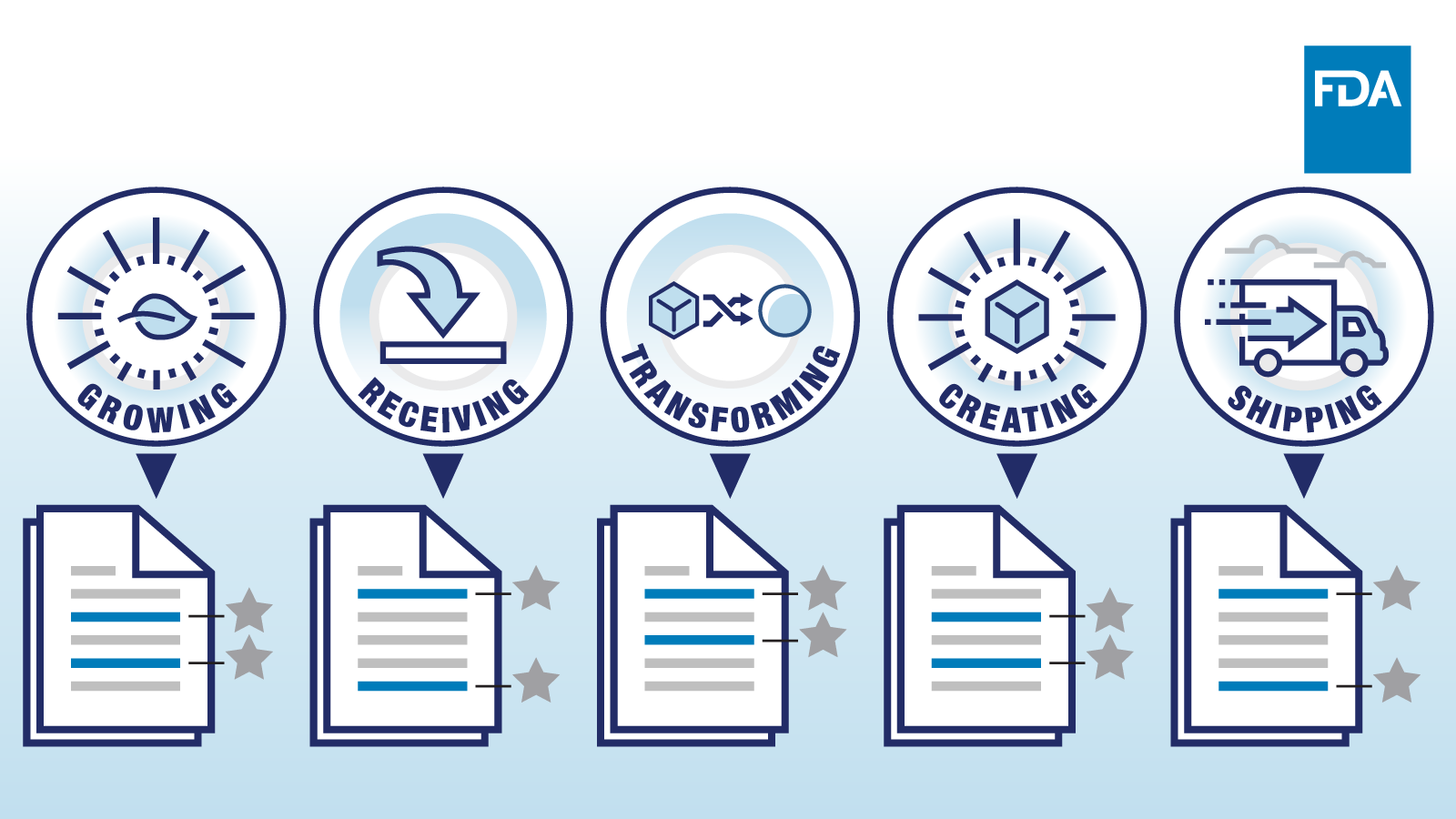
Fda S Proposed Food Traceability Rule The Basics National Agricultural Law Center

Solved Branches Of Government That Is Legislative Chegg Com

The Food And Drug Administration Safety And Innovation Act Fdasia P L 112 144 Everycrsreport Com